TECHNOLOGY DESCRIPTION
TECHNOLOGY:
The Italian research team coordinated by dott. Sara Marinelli (CNR - Cell Biology and Neurobiology Institute) is focusing its research on pain transmission and inflammatory and chronic pain modulation, achieving important and innovative results in using of toxins of bacterial origin as new drug with new therapeutic use.
The recent studies have led the Italian group to analyzing the Botulinum neurotoxin type A (BoNT/A), a potent biological toxin widely used in clinical practice for treatment of various disorders such as dystonia, including muscle spasticity, headaches and overactive bladder disorders.
The Italian researchers have demonstrated that BoNT/A is able to contrast neuropathic pain (Nep) caused by peripheral nerve injury, and have patented a novelty in the treatment for spinal injuries and symptoms associated with them, from pain to paralysis. The invention demonstrates that, in a mouse model, BoNT/A is an efficacious treatment for a complete recovery from paralysis (paraplegia/tetraplegia) and restoration of sensitivity.
EXPERTISE
The inventor’s lab has mainly focused its research on pain transmission and inflammatory and chronic pain modulation, achieving important and innovative results in this field:
a) use of toxins of bacterial origin,
b) new drugs with new therapeutic use,
c) knock-out or transgenic mice for specific proteins involved in nociception or that reproduce a genetic pathology.
Three main goals are pursued:
1) study the mechanisms that rule the information along the nervous system appointed to pain transmission and control,
2) investigate the degeneration processes associated to neuropathies,
3) identify new pharmacological treatments in a therapeutic perspective.
BENEFITS
Spinal cord injury occurs throughout the world with an annual incidence of 15 to 40 cases per million. The causes of these injuries range from motor vehicle accidents and community violence, to recreational activities and workplace-related injuries. The incidence of spinal cord injury is greater among young men (80% of all spinal cord injuries are sustained by men at the average age of 40 years).
People with spinal cord injuries are living with devastating disabilities, experience chronic pain and an estimated significant percentage show signs of depression, causing detrimental effects on their quality of life and a considerable financial burden on society. Lifetime health care costs associated with spinal cord injuries are expensive and vary according to age at the time of injury and extent of injury. Estimated expenses range from approximately $700,000 to care for a 50-year-old patient who suffers paraplegia to >$3 million to care for a 25-year-old sustaining a high tetraplegia (C1-C4) throughout the course of their lives, excluding loss of wage and productivity.
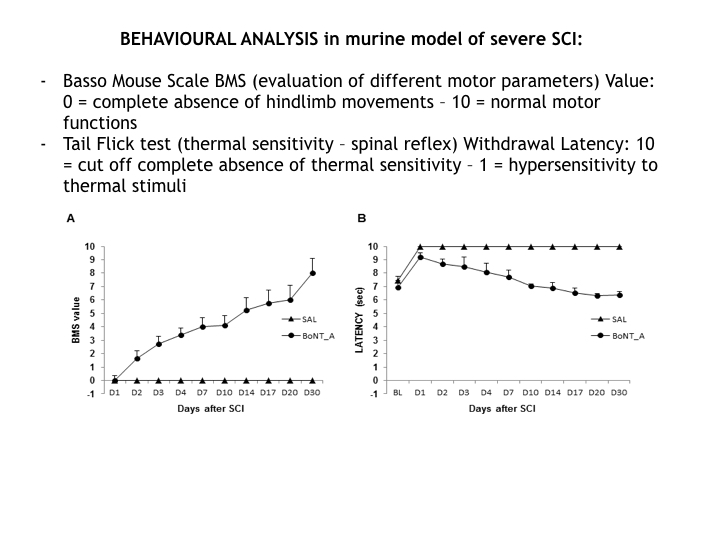
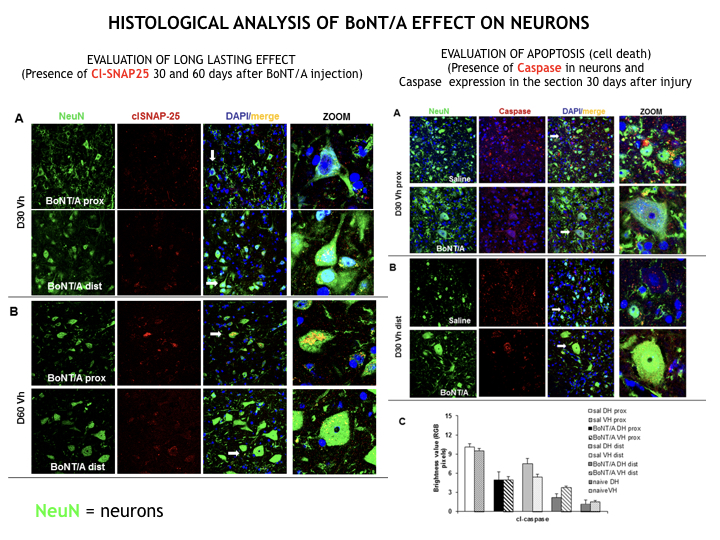
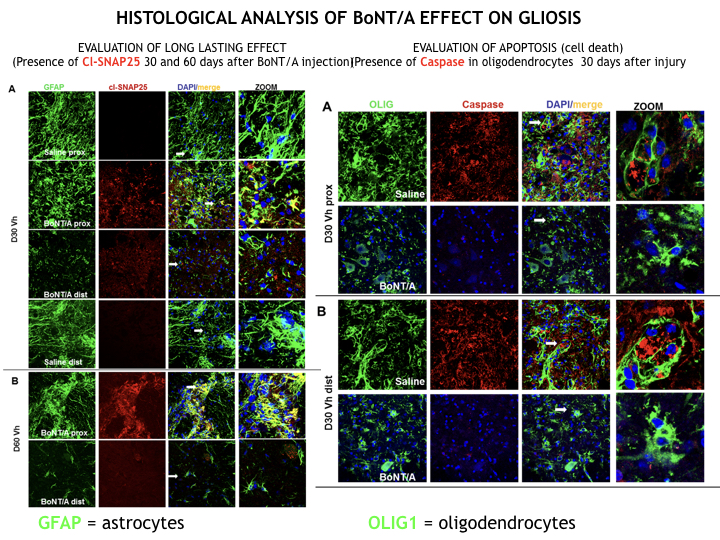
To date, effective neuroprotective therapies in the care of spinal cord lesion don’t exist. We have discovered the great pro-regenerative action of BoNT/A that, when directly infused in spinal cord (intrathecal administration), dramatically reduces local inflammation, blocking neuronal death, and induces nervous regeneration and remyelination. We observed in a clinically relevant mouse model of SCI a complete restoration of motor function and sensitivity.
Its unique feature of long-lasting effect, a single dose is able to act for months, represents a formidable advantage. BoNT/A constantly exerts its powerful anti-inflammatory effect, reducing glia scar formation and counteracting cell death phenomena due to excitoxicity; processes that begin early after lesion and persist for months, leading to a point of no return.
We observed a progressive regression of pathology in mice treated with BoNT/A when compared with placebo. Motor function, muscle atrophy, sensitivity, spinal reflex were visibly recovered, neuropathic pain was reduced.
We offer a NEW therapeutic use of BoNT/A already widely used in clinical practice. This greatly reduces the time for translation to patients because kinetic, toxicity and dose/response curve are known. Furthermore, there are already several commercial formulations for the administration of BoNT/A. No side effects at therapeutic doses have been registered.
LIMITATIONS
Not Known
APPLICATIONS
Recovery from paralysis (paraplegia/tetraplegia) and restoration of sensitivity within the treatment of peripheral nerve injury.
MATERIALS
Readiness Level (TRL)
 Patent Grading Report |
Patent Grading ReportThe Grading Patents Report evaluates and grades US patents Sample Buy from Wisdomain |
STATUS
Current status
PCT Application
INVENTOR / TEAM
Dr. Flaminia Pavone, Dr. Siro Luvisetto, Dr. Sara Marinelli, Dr. Valentina Vacca, Neuroscience and Pain Lab, CNR - Cell Biology and Neurobiology Institute.